For more background on what Soluble Solutions and Irv Wiehe offers in Resid Conversion please read the following tutorial on the subject.
Some of the key points are:
+Conversion is limited by polynuclear aromatics.
+Coke formation can be delayed by keeping asphaltenes in solution.
+The Pendant-Core Model enables understanding Conradson Carbon and how it changes with processing.
+Present Resid Conversion Processes can be greatly improved.
+Reduce formation of polynuclear aromatics.
+Reduce hydrocarbon gas formation.
+Eliminate fouling by coke and sediments.
+Use effective combinations of separations and conversion.
+Optimize process conditions to the resid being processed.
+Process Models require kinetics, resid stoichiometry, vapor-liquid equilibria, liquid-liquid equilibria, and reactor model.
Resid Conversion Tutorial
Introduction
Petroleum resids are the heavy fraction remaining after distilling petroleum crudes at atmospheric pressure (atmospheric resid) or at reduced pressure of 25 to 100 mm Hg (vacuum resid). Not only do resids have high molecular weight (About 1000 amu number average for vacuum resids) but they contain polynuclear aromatics (PNA’s). The PNA’s greater than 3-4 rings provide the greatest limitation to the conversion of resids. This is because of their high thermal stability and because desirable aromatics in transportation fuels are limited to alkylated single rings. In addition, the high concentrations of heteroatoms (sulfur, nitrogen, vanadium, and nickel) in petroleum resids act along with PNA’s to poison catalysts. Therefore, care must be taken to protect catalysts, to replace and regenerate catalysts, to use throwaway catalysts, or to use purely thermal processes. No matter which type of process is used, a substantial fraction of resid molecules can be cracked off as fragments to become liquids in the transportation fuel and vacuum gas oil boiling ranges. However, one should not try to overly convert resids or the PNA limit will force the selectivity to go to the thermodynamically favored (1) but lower valued products: coke and/or hydrocarbon gases. As a result, all economical resid conversion processes have an even heavier carbonaceous byproduct. It is critical to have an economical and environmentally acceptable use for this byproduct for the resid conversion process to be viable.
Characterization of Resids
The understanding of the chemical transformations of the macromolecules during resid conversion is greatly limited by the wide diversity (over a million chemical structures) of these complex macromolecules. One way to sample this molecular diversity is to separate the resid and its conversion products into diverse fractions using solubility/insolubility in volatile liquids as well as adsorption/desorption on solids. In this way a number of resids and resid conversion products were separated into coke (toluene insoluble), asphaltenes (toluene soluble/n-heptane Insoluble), resins (n-heptane soluble/adsorbs on Attapulgus clay), small ring aromatics (n-heptane and cold methylethyl ketone soluble/does not adsorb on Attapulgus clay), and saturates (n-heptane soluble/ cold methylethyl ketone insoluble/does not adsorb on Attapulgus clay). A plot of molecular weight versus hydrogen content, the solvent-resid phase diagram in Figure 1, shows that each of these fractions occupies an unique area that better defines the fraction than the separation method(2). Thus, to a first approximation resids differ in the relative amounts of similar fractions: saturates, small ring aromatics, resins, and asphaltenes (unconverted resid contains no coke).
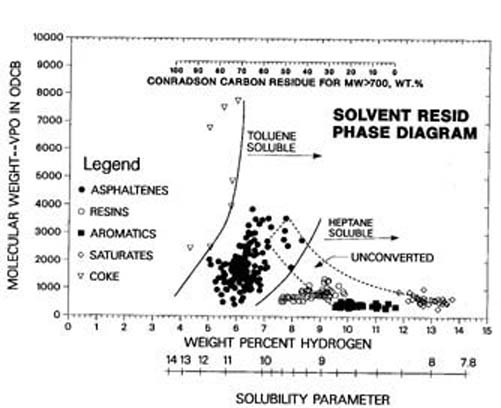
Figure 1. The Solvent – Resid Phase Diagram
While saturates and small ring aromatics contain sulfur, they contain little or no nitrogen, oxygen, vanadium, nickel, or PNA’s of 3 rings or larger (2). Thus, they are relatively catalyst friendly. During conversion, the saturates can be completely converted to lower molecular products without forming a carbonaceous byproduct. However, the small ring aromatics combine during thermal cracking to form resins. The resins crack off side chains from PNA’s to form lower molecular weight, lower hydrogen content byproducts. When these PNA containing molecules combine, they form higher molecular weight asphaltenes. The asphaltenes crack off side chains from PNA’s to form lower molecular weight, lower hydrogen content byproducts until they combine to form higher molecular weight coke (2). The addition of hydrogen and the presence of catalysts can alter the rates of these aromatic combination reactions and thus the selectivity to products. However, at high enough conversion, coke and gas are the principal products, independent of conversion chemistry as the remaining molecules approach partially saturated PNA’s with very short side chains.
Hydrocarbon Solubility
At low temperatures saturates can have lower solubility than small ring aromatics because of the formation of crystalline waxes. However, above the wax melting point (40 to 60°C) where resids are processed, all fractions become less soluble in hydrocarbons with lower hydrogen content and higher molecular weight (3). Figure 1 shows this with the solubility parameter increasing with lower hydrogen content (higher aromaticity) because the attractive force between PNA’s is the strongest intermolecular force in resids and resid conversion products. This is very important in the mechanism of coke and sediment formation that often plagues resid conversion processes.
Pendant-Core Building Block Model and Conradson Carbon Residue
Models of petroleum with detailed chemical structures are misleading with the present characterization uncertainty and lead to overly complex kinetic models for the predictive power. However, one does not approximate reality if the wide diversity of molecular types is not included. A compromise that satisfies both of these requirements is to represent molecules as combination of pseudo building blocks. The simplest model has two building blocks(4). Since thermally stable polynuclear aromatics are such an important part of the mechanism of coke formation, they are represented by the aromatic core building block. The other building block represents the fragments that become volatile liquids when cracked off the core, called pendants. Then a vacuum resid can be represented by a wide distribution of species made up of various combinations of these two building blocks (Figure 2). If each building block is assigned a hydrogen content and a molecular weight, the diversity of the solvent-resid phase diagram (Figure 1) will be represented.
Conradson carbon residue (CCR) is a standard petroleum industrial coking test for characterizing the coke forming tendency of petroleum liquids that today is measured by the Microcarbon method(5) (ASTM D4530). Since this test is widely used, it is extremely important for those working in resid conversion to understand what this test does or does not reveal. In the terms of the Pendant-core Building Block Model, CCR measures the percent fraction of the resid that is cores. A hydrogen balance around the Microcarbon test (Figure 3), shows that the CCR is a linear function of hydrogen content, a previously unexplained result (6, 7). This enables determining that the hydrogen content of the core is 3.8 wt.% and the hydrogen content of the pendant is 11.6 wt.% (Figure 4). These values were independently verified by directly measuring the hydrogen content of the coke in the microcarbon test and the conversion independent hydrogen content of distillable liquid product of vacuum resid thermolysis(4). In addition, the pendant-core model explains why CCR is conserved for physical separations (hydrogen is conserved), increases for thermal conversion (hydrogen escapes as part of hydrocarbon gas byproduct), and decreases for hydroconversion (hydrogen is added).
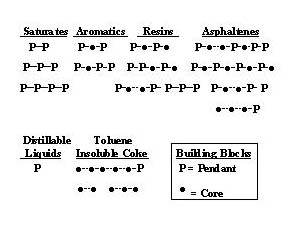
Figure 2. The Pendant – Core Building Block Model Allows for a
Wide Distribution of Species
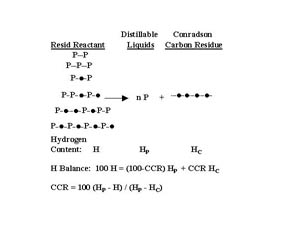
Figure 3. Model Shows Conradson Carbon Residue is a
Linear Function of Hydrogen Content
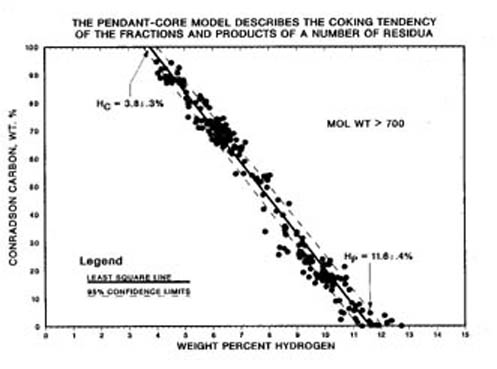
Figure 4. Conradson Carbon is a Linear Function of Hydrogen Content
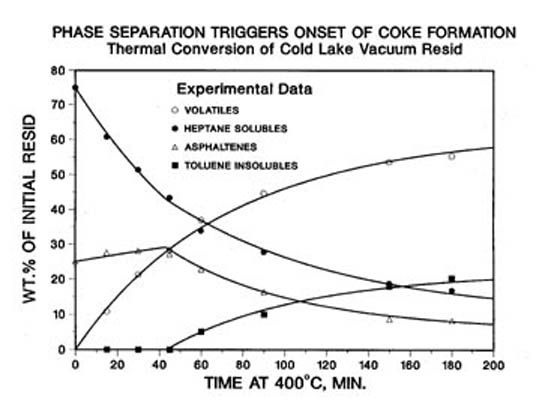
Figure 5. Kinetic Data Has a Coke InductionPeriod when Asphaltenes Are Soluble
Selection of Resid Conversion Processes
Most petroleum resid processes can be made up of combinations of separations, thermal conversion, hydroconversion or hydrotreating, and fluid catalytic cracking. Distillation is the most common separation choice because of its low cost. However, fuels deasphalting is becoming more popular because it can separate the saturates and small ring aromatics out of resids. Nevertheless, the selection of the conversion process depends on the quality of the resid or the selection of the crude for the refinery depends on the resid conversion process. Resids high in saturates and small ring aromatics and low in resins and asphaltenes are more appropriate for catalytic processing. On the other hand, resids high in resins and asphaltenes are more suited for coking.
Coking
In coking processes the resid is heated to high temperatures (450-500�C) with the light boiling products distilling overhead and leaving the solid coke behind. Coking is the most popular conversion choice for resids high in cores (low hydrogen, high heteroatoms, and high Conradson carbon) because it reactively separates the cores, the catalyst poisons, into a low value byproduct (coke) leaving the pendants for catalytic upgrading. Therefore, coking should be called a “catalyst poison rejection” process because a principal reason for coking is to improve the liquids for further upgrading by catalytic processing(8). Unfortunately, coking has been mistakenly called a “carbon rejection” process, a term that continues to be repeated without critical examination. For example, Table I shows that the coke product in a microcarbon tester has a much higher concentration of sulfur, nitrogen, and metals than the vacuum resid feed and by atomic balance, the volatile liquid products. However, the concentration of carbon in the vacuum resid, in the coke, and in the liquid product is nearly the same. The rejection of heteroatoms is counter balanced by an increase of hydrogen content in the liquid product. This highly desirable result is because most of the polynuclear aromatic building blocks in the petroleum macromolecules contain heteroatoms that are reactively separated with the polynuclear aromatics into the coke.
Kinetics of Coke Formation and Mesophase
The thermal cracking of resid is a first order reaction with a constant activation energy over a large range of temperatures(9). There is an induction period before coke begins to form (Figure 5). It is now understood (10) that coke formation is triggered by the phase separation of a second liquid phase formed from partially converted asphaltene cores. Evidence of the liquid – liquid phase separation of asphaltene cores, promoted by the stacking of polynuclear aromatic structures, is the presence of spherical liquid crystalline coke (10), called the carbonaceous mesophase. When carbonaceous mesophase is built up from the thermolysis of 2-3 ring aromatics, i.e. fluid catalytic cracker bottoms, it remains fluid enough to be spun into fibers and than carbonized at very high temperatures after crosslinking with oxygen (11). Alternatively, the carbonaceous mesophase can form needle coke in delayed cokers from similar feeds. However, the asphaltene cores from resids are so reactive that their mesophase solidifies before a high degree of ordering can happen. Nevertheless, the presence of mesophase in a coke sample can be used as a diagnostic tool to show that it was caused by thermal cracking and insolubility at high temperatures (12).
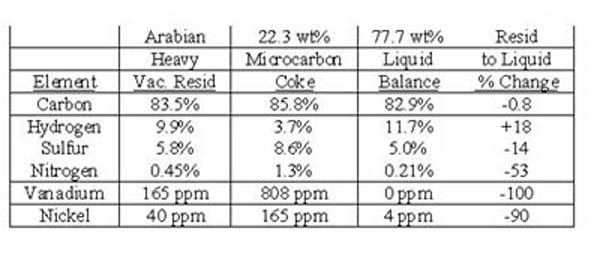
Table I
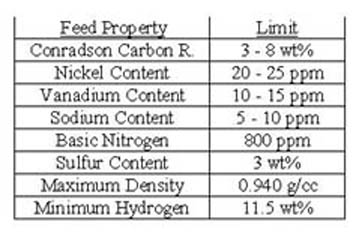
Table II
Delayed Coking (13)
The delayed coking process is the oldest and most popular choice. The resid is heated by flowing through a long tube in a furnace and then reacted by flowing into the bottom of a high, cylindrical, insulated drum. The volatile products go overhead into a fractionator and coke accumulates in the drum. The heavy liquid product from the fractionator is recycled back to the furnace. Meanwhile, when the drum fills up with coke the feed is switched to a second drum. The coke is mined out of the drum by drilling a hole down the center with high pressure water and cutting out the remainder also with high-pressure water to get the drum ready for the next 16 hour reaction cycle. Anode grade coke for making electrodes for manufacturing aluminum commands a higher price than fuels grade coke. However, the low specification on sulfur, vanadium, and nickel content for the coke requires a higher quality (high in saturates and small ring aromatics) and more expensive resid feed. Needle coke for making electrodes for steel manufacturing is priced even higher ($500/ton) than anode grade coke but it requires low sulfur, fluid catalytic cracker bottoms, not resid, as a feed.
Fluid Coking and FLEXICOKING (14)
These processes are licensed by Exxon. In Fluid Coking the resid is sprayed on a hot, fluidized bed of coke particles in a vessel, the reactor. The volatile products go overhead to a fractionator while the coke particle are removed out of the bottom and transferred to another vessel, the burner, where the coke is partially burned with air to provide the heat for the process. This coke then is recirculated back to the reactor. Since this process produces much more coke than is required for heat, fluid coke is withdrawn at the bottom of the reactor. This is a high sulfur, fuels grade coke of low value. A heavy liquid product is recycled back to the reactor from the fractionator. This is used to scrub coke fines from the reactor vapors and to improve the quality of the liquid product. In FLEXICOKING a third vessel, the gasifier, is added to the Fluid Coker. In the gasifier coke is gasified with steam and air in net reducing conditions to produce a low BTU gas containing hydrogen, carbon monoxide, nitrogen, and hydrogen sulfide. After adsorbing out the hydrogen sulfide, this low BTU gas is burned as a clean fuel within the refinery and/or in a nearby power plant. Only a small amount of coke (about 3%) needs to be withdrawn in FLEXICOKING to keep vanadium and nickel from accumulating in the gasifier. With the trend of decreasing high sulfur coke prices, gasification of coke to form fuel and syngas to be used within the refinery may be the only economical and environmental option for refineries in the future. If this happens, FLEXICOKING may become a more popular choice.
Visbreaking
This is a low conversion thermal process used originally to reduce the resid viscosity for heavy fuel oil applications. However, today it uses a resid that exceeds minimum heavy fuel oil specifications and converts just enough to obtain 15-30% transportation boiling range liquids and still have the heavy product meet heavy fuel oil specifications. Since this process cannot tolerate coke formation, it is required to be within the coke induction period that may limit conversion, rather than heavy fuel oil specifications. A visbreaker reactor may be similar to a delayed coker with a furnace tube followed by a soaker drum. However, the drum is much smaller in volume to limit the residence time with the entire liquid product flowing overhead. Alternatively, the entire visbreaker may be a long tube coiled within a furnace. Upsets cause coke to form and accumulate on visbreaker walls so that they need to be decoked periodically.
Hydroconversion
Hydroconverion combines thermal cracking with hydrogenation. The addition of hydrogen increases the coke induction period by lowering the solubility parameter by hydrogenating PNA’s but primarily by terminating free radicals and reducing the frequency of aromatics from combining to form larger PNA’s. Thus, hydroconversion of vacuum resids to volatile liquids can be over 85% as opposed to 50-60% for coking. However, one has to deal with the cost of hydrogen and catalyst, high-pressure vessels, poisoning of catalysts, the difficulty of asphaltenes to diffuse through small pores, and the intolerance to coke and sediment formation. The active catalyst needs to be a transition metal sulfide because the high level of sulfur in resid feeds will poison other hydrogenation catalysts.
Fixed Beds
Since metals removal is one of the fastest reactions and since the metals accumulate in the pores of supported catalysts, it is common to have a guard bed in front of the fixed bed. When insufficient demealization activity occurs in the guard bed, the feed is switched to a second guard bed with fresh catalyst and the catalyst is replaced in the first guard bed. Thus, the fixed bed is protected from metals deposition. In order to hydrogenate the largest macromolecules in the resid, the asphaltenes, some or all of the catalysts need to have pores 50 to 100 microns in diameter. Even with these precautions it is difficult to get longer than one year run lengths on fixed bed hydrocoversion units with vacuum resid feeds and conversions to volatile liquids of 50% or more. This is because of catalyst deactivation with coke or by coke and sediment formation downstream of the reactor.
Ebulating Beds
The LC Finer and H-Oil units mechanically ebulate the catalyst so that it can be mixed and replaced onstream. The conversion is greatly dependent on the feed but conversions of vacuum resids to volatile liquids of the order of 70% are possible. Often these units are limited by the deposition of coke and sediment downstream of the reactor in hot and cold separators(15).
Dispersed Catalysts(16)
If one cannot diffuse the asphaltenes to the catalyst, why not diffuse the catalyst to asphaltenes. Dispersed catalysts also can be continuously added in low enough amounts (i.e. 100 ppm) to consider them throwaway catalysts with the carbonaceous by-product. However, economics usually dictate some form of catalyst recycle to minimize catalyst cost. Nevertheless, by designing the reactor to maximize the solubility of the converted asphaltenes, the conversion of vacuum resids to gas and volatile liquids can be above 95% with greater than 85% volatile liquids. However, the last 5 to 10% conversion may not be worth the cost of hydrogen and reactor volume to produce hydrocarbon gases and very aromatic liquids from this incremental conversion. The answer depends on the value and use of the unconverted carbonaceous liquid byproduct (essentially all PNA cores).
Resid Catalytic Cracking(17)
Resid catalytic cracking has much better selectivity to desired products (high gasoline and low gas yields) than for coking or hydroconversion. In fluid catalytic cracking feed is sprayed on zeolite catalyst in a short contact time, riser reactor. The vaporized product flows to a fractionator while the catalyst with coke and adsorbed hydrocarbons flow to a fluidized bed regenerator where the coke and hydrocarbons are burned off the catalyst. Fluid catalytic cracking requires much higher quality feeds than coking or hydroconversion. This is because of expensive zeolite catalysts, intolerance to sodium, nickel, vanadium, and basic nitrogen as well as limitations on the amount of coke that can be burned in the regeneration step by cooling capacity. As a result, the feed in resid catalytic cracking is at worst an excellent quality atmospheric resid but mixtures of vacuum gas oil and atmospheric resids are more common. The total feed is limited to Conradson Carbon Residues of 3 to 8 wt.% , depending on the cooling capacity. This and other typical feed limitations are found in Table II(17).
Future Improvements of Resid Conversion Processes
We are still on the steep part of the learning curve as to the characterization, phase behavior, and conversion chemistry of petroleum resids. As a result, much room remains to improve resid conversion processes. However, the rate of construction of new resid conversion units in refineries has been decreasing. The future growth appears to be at or near heavy crude production sites to decrease heavy crude viscosity and improve the quality to ease transportation and open markets for crudes of otherwise marginal value. There remains room for improving coking and hydroconversion processes by reducing hydrocarbon gas formation, by inhibiting the formation of PNA’s not originally present in the resid, and by separating an intermediate quality (low in cores) fraction before or during conversion. Both of these processes would benefit if a higher valued byproduct, such as carbon fibers or needle coke, could be formed from the PNA’s. In addition, the challenge for hydroconversion is to take advantage of the nickel and vanadium in the resid to generate an insitu dispersed catalyst and to eliminate catalyst cost. Finally, resid catalytic cracking needs to move to poorer quality and lower cost feeds by making more tolerant catalysts, by processing only the saturates and small ring aromatics portion, and/or by improved methods to remove heat from the regenerator.
LITERATURE CITED
1. Fitzer, E., Mueller, K., Schaefer, W., in “Chemistry and Physics of Carbon”, P. L. Walker, ed., Marcel Dekker, 7, 237 (1971).
2. Wiehe, I.A.,Ind. Eng. Chem. Res., 31, 530 (1992).
3. Wiehe, I.A., Liang, K.S., Fluid Phase Equilibria, 117, 201 (1996) and Fuel Sci. & Tech. Int’l, 14, 289 (1996).
4. Wiehe, I.A., Energy and Fuels, 8, 536 (1994).
5. Noel, F., Fuel, 63, 931 (1984).
6. Roberts, I. Prepr. Pap.- ACS, Div. Pet. Chem., 34, 251 (1989).
7. Green, J.B. et al., Energy and Fuels, 6, 836 (1992).
8. Wiehe, I. A., ACS Div. Pet. Chem. Meeting, Chicago (1995).
9. Olmstead, W. N., Freund, H., AIChE Spring National Meeting, New Orleans, 1998.
10. Wiehe, I.A., Ind. Eng. Chem. Res., 32, 2447 (1993).
11. Diefendorf, R.J., in “Petroleum Derrived Carbons”, M.L. Deviney and T.M. O’Grady, ed., ACS Symposium Series, 21, 215 (1976).
12. Wiehe, I. A., ACS Div. Pet. Chem. Meeting, San Francisco (1997).
13. Ellis, P. J. and Paul, C.A., AIChE, International Conference on Refinery Processes, Preprints, ed. I.A.Wiehe, 151 (1998).
14. Hammond, D.G., AIChE, International Conference on Refinery Processes, Preprints, ed. I.A.Wiehe, 170 (1998).
15. Nowlan, V.J. and Srinivasan, N.S., Fuel Sci. & Tech. Int’l, 14, 41 (1996).
16. Bearden, R. and Aldridge, C.L., US Patent 4,134,825 (1979) and ACS Div. Pet. Chem. Meeting, ACS Award in Petroleum Chemistry Address, San Francisco (1997).
17. Barnes, P.H., AIChE, International Conference on Refinery Processes, Preprints, ed. I.A.Wiehe, 227 (1998).
